
Cortland Biomedical’s newly designed, purpose-built manufacturing has been strategically designed by textile engineers who truly understand the ins and outs of creating advanced medical textile constructions and have carefully evaluated input from our customers about their needs.
![]()
Facilities and Equipment
Cortland Biomedical’s newly designed, purpose-built manufacturing has been strategically designed by textile engineers who truly understand the ins and outs of creating advanced medical textile constructions and have carefully evaluated input from our customers about their needs.
![]()
ISO Class 8 and ISO Class 7 Facilities for Biomedical Textile Assembly, Cleaning, and Post-Processing
We have greatly expanded from our clean room space, with ISO Class 8 clean rooms for fabric formation and ISO Class 7 clean rooms for assembly, cleaning and post-processing.
We operate under the design and control guidance of ISO 13485:2016 and our Quality Management System is compliant to 21 CFR part 820. We work closely with you to ensure documentation generated meets product performance required to support your FDA or European filing.
Facilities
We have greatly expanded from our clean room space, with ISO Class 8 clean rooms for fabric formation and ISO Class 7 clean rooms for assembly, cleaning and post-processing.
We operate under the design and control guidance of ISO 13485:2016 and our Quality Management System is compliant to 21 CFR part 820. We work closely with you to ensure documentation generated meets product performance required to support your FDA or European filing.
Additional Capabilities

Precision Winding
We’ve incorporated the latest in precision tension control into our winding processes. Digital traverse, servo motor feeds and on-board load cells capable of delivering tension control to 0.1 gram are part of our commitment to offering you the latest in textile technology.

Aqueous Scouring
We’ve designed a fully integrated water purification system for removing manufacturing finishes and lubricants. We routinely perform microbial and endotoxin testing on water taken from sanitary sampling ports placed at strategic locations throughout our facility.
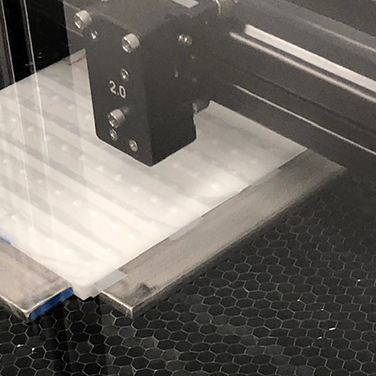
CO2 Laser Cutting
We offer a near-net-shaped component engineered to integrate seamlessly into your finished device design. Our precision laser cutting equipment offers the ability to cut specific shapes, pre-drill suture and fixation points, and seal the edges of polymeric fabrics to eliminate fraying.
Featured Equipment

Precision Winding
We’ve incorporated the latest in precision tension control into our winding processes. Digital traverse, servo motor feeds and on-board load cells capable of delivering tension control to 0.1 gram are part of our commitment to offering you the latest in textile technology.

Aqueous Scouring
We’ve designed a fully integrated water purification system for removing manufacturing finishes and lubricants. We routinely perform microbial and endotoxin testing on water taken from sanitary sampling ports placed at strategic locations throughout our facility.
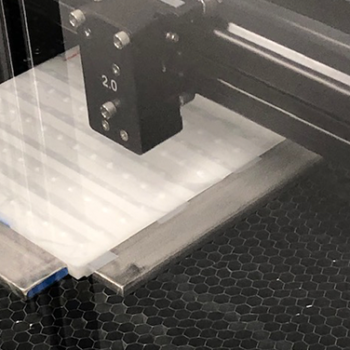
CO2 Laser Cutting
We offer a near-net-shaped component engineered to integrate seamlessly into your finished device design. Our precision laser cutting equipment offers the ability to cut specific shapes, pre-drill suture and fixation points, and seal the edges of polymeric fabrics to eliminate fraying.









